Predictors of Device-Related Thrombus Following LAA Occlusion
Thrombus formation has emerged as the Achilles’ heel of this intervention—a new score may help with risk prediction.
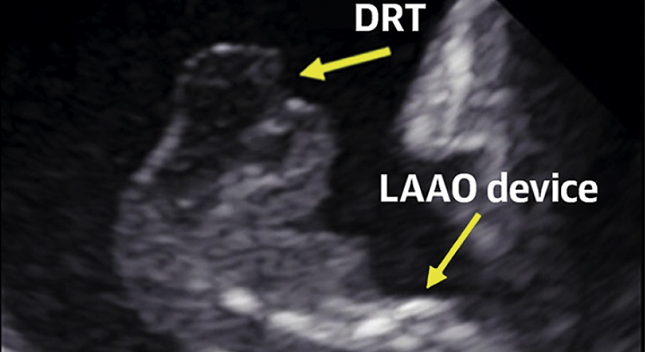
(UPDATED) Device-related thrombus (DRT) following left atrial appendage (LAA) occlusion is associated with subsequent ischemic events, but a range of patient- and procedure-related factors may help physicians to identify patients at risk for this complication.
So concludes an analysis of cases collected by an international DRT registry that was led by Trevor Simard, MD (Mayo Clinic, Rochester, MN), and published today in the Journal of the American College of Cardiology. The majority of devices included in the registry were first-generation Watchman device (Boston Scientific).
“This is considered sort of the Achilles’ heel of this therapy so far,” Mohamad Alkhouli, MD (Mayo Clinic), told TCTMD. The chief aim of LAA closure is to prevent thrombus development and stroke, so the finding that device-related thrombi were turning up in a small proportion of patients was a setback for the field. Most of the research to date, Alkhouli added, has been focused on quantifying the problem, addressing the temporal evolution, and exploring options for treatment.
This particular registry study, LAAO-DRT, was undertaken to try to identify patient- or procedure-related factors that could help predict the risk of DRT forming in the first place, Alkhouli said.
International Collaboration
The LAAO-DRT registry included 711 patients from 37 international centers, among them 237 who had developed DRT anywhere from day 1 to more than 1 year after device implantation. Nearly one-quarter of these patients developed their thrombus in the first 45 days, nearly 39% between days 45 to 180, 16% between 180 and 365 days, and 20% beyond 1 year (median follow-up was 1.8 years). At last known follow-up, one-quarter of the patients had DRT.
The timing is worrisome, Alkhouli stressed. “Almost three-fourths of all the DRTs happened beyond the 45 days, which is when we currently tell patients they can stop the blood thinner, based on the [indications for use].” At least two patients developed DRT after the 2-year mark, he added.
“Some people would say, ‘Well, if you look more, you'll find more,' but that's that doesn't take away from the fact that if you don't look, it doesn't mean that it's not there,” he said. “So the fact that DRT happens late remains concerning.”
After multivariable analysis, the dominant risk factors for DRT were hypercoagulability disorder (OR 17.50; 95% CI 3.39-90.45) and pericardial effusion (OR 13.45; 95% CI 1.46-123.52), followed by renal insufficiency (OR 4.02; 95% CI 1.22-13.25), implantation depth greater than 10 mm from the pulmonary vein limbus (OR 2.41; 95% CI 1.57-3.69), and nonparoxysmal atrial fibrillation (OR 1.90; 95% CI 1.22-2.97).
Pinpointing these factors allowed investigators to come up with a risk score that they believe can help inform patient-physician discussions prior to invasive treatment. “We were successful in identifying several factors that can lead to device thrombosis: some of them are modifiable, some of them are not. We cannot modify renal insufficiency or permanent A-fib, but the device implant we can,” Alkhouli said. “Then, if you have two of those five risk factors, then you have a twofold increase—a hundred percent increase—of risk of a DRT. So maybe if we have a patient with a borderline indication or we're not certain if this is the right therapy to do for a patient, then maybe we should consider the risk of DRT when we do the shared decision-making.”
The score needs to be prospectively validated, he added.
Importantly, presence of DRT was associated with a higher risk of death, ischemic stroke, or systemic embolization (HR 2.37; 95% CI 1.58-3.56), which according to Simard and colleagues was principally driven by the risk of ischemic stroke.
Patients diagnosed with DRT are typically managed with intensive (or intensified) antithrombotic agents. “This approach successfully clears thrombus in many cases with only a quarter of cases demonstrating persistent DRT presence in follow-up,” the authors write. “Reassuringly, despite the bleeding risk of these patients, our studied cohort did not suggest an increase in bleeding rates with the treatment of DRT.”
But one of the important contributions of this analysis, said Alkhouli, was that presence of DRT was not associated with discharge medications, which ranged from single and dual antiplatelet therapy to oral anticoagulation (OAC) alone, OAC plus single antiplatelet therapy, and OAC plus heparin. In fact, medical regimens were similar between patients who did and did not have DRT. Most patients were being managed with single (36%) or dual antiplatelet therapy (26%) at the time of DRT diagnosis.
“There is this whole conversation or debate about should we do short-term blood thinners, or should we do dual antiplatelet therapy, or does it matter if we do anything at all?” said Alkhouli. He and his colleagues previously showed in a meta-analysis that antithrombotic regimen did not explain the heterogeneity of DRT formation after LAA closure. “Here we found the same thing. We found that there was no difference no matter what the medication was, so that was a surprise,” Alkhouli said.
Predicting DRT
Commenting on the study for TCTMD, Vivek Reddy, MD (Icahn School of Medicine at Mount Sinai, New York, NY), called the paper a “very important contribution to the field,” particularly since it reflects such a broad, international experience with the device. It also mirrors the 3% to 4% risk of DRT that has been reported elsewhere, he noted. Using the predictors identified to come up with a score that could be applied to patients prior to device implantation is also potentially very helpful, Reddy said.
“For example, having a hypercoagulability disorder certainly does seem to put patients at a very high risk. So you'd have to think really hard about whether or not that's the kind of patient you'd want to put this [device] in, because if they develop device related thrombus they're going to be dealing with that for quite some time,” Reddy told TCTMD. “I think this could inform our practice.”
The timing of DRT diagnosis also is revealing, he noted, since it points to the fact that it is not confined to a specific early window—a point made by Alkhouli as well. When the Watchman LAA closure device was first approved, the indications for use recommended 6 weeks of OAC, with cardiac imaging (transesophageal echo [TEE] or cardiac CT) at 6 weeks. What Reddy does in practice, however, has shifted to being 6 weeks of OAC, followed by 6 weeks of DAPT and then 6 weeks of low-dose aspirin, with follow-up imaging at the 4-month mark.
“So the idea is, we've waited long enough so that if a clot's going to form it would have formed already, but we're not waiting so long that it might break off, and then we do our first imaging,” he said. “The idea of doing a TEE at 6 weeks is, in my mind, a bit antiquated, with the current antithrombotic regimen.”
Both Reddy and Alkhouli made the point that manufacturers have also been working on device improvements to reduce thrombosis risk, include trying to minimize the amount of exposed metal or introducing antithrombotic surfaces and coatings. According to Reddy, DRT rates on the new Watchman FLX device are closer to 1.8%. “So it’s not gone, but it’s certainly a lot better and maybe half as frequently as it was happening [with the earlier generation Watchman],” said Reddy. “The question of course is: what do you coat with or what are you trying to do? And that's an area that still needs a lot of work.”
In an accompanying editorial, Oussama Wazni, MD (Cleveland Clinic, OH), and colleagues point out that the issue of DRT will only grow in importance as LAA closure expands to lower risk and younger patients. “Such growth warrants particular attention to unresolved issues and ensuring long-term safety,” they write. And while device innovation has appropriately focused on reducing risks, “the benefits from such strategies have yet to be determined.”
More insights are expected from the European Society of Cardiology Congress in late August, where results from the more than 1,800-patient randomized trial comparing the investigational Amulet left atrial occluder (Amplatzer) to the Watchman will be presented during a hot-line session.
“As the field grows wider, enhancing LAA occlusion safety with optimal design, implantation, and periprocedural management is critically important; yet, the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Simard T, Jung RG, Lehenbauer K, et al. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol. 2021;78:297-313.
Wazni, Saliba W, Hussein AA. Device-related thrombus after left atrial appendage occlusion. J Am Coll Cardiol. 2021;78:314-316.
Disclosures
- Simard reports no relevant conflicts of interest.
- Alkhouli reports serving as a consultant for Boston Scientific.
- Reddy reports consulting with Abbott, Axon, Biosense-Webster, Biotronik, Boston Scientific, Cardiofocus, Cardionomic, CardioNXT/AFTx, EBR, Impuse Dynamics, Medtronic, Philips, Stimda, and Thermedical; holding equity in Manual Surgical Sciences, Newpace, Surecor, and Vizaramed; and both consulting and equity with Ablacon, Acutus Medical, Affera, Apama Medical, Aquaheart, Atacor, Autonomix, Backbeat, BioSig, Circa Scientific, Corvia Medical, Dinova-Hangzhou Nuomao Medtech, East End Medical, EPD, Epix Therapeutics, EpiEP, Eximo, Farapulse, Fire1, Javelin, Kardium, Keystone Heart, LuxCath, Medlumics, Middlepeak, Nuvera, Sirona Medical, and Valcare.
- Wazni reports receiving research grant support from and consulting for Boston Scientific.




Comments