Prior Transradial Cath Shouldn’t Mean Radial Graft a No-go for Surgery
Cardiac cath with radial access usually makes the artery out-of-bounds for surgery—that should change, say researchers.
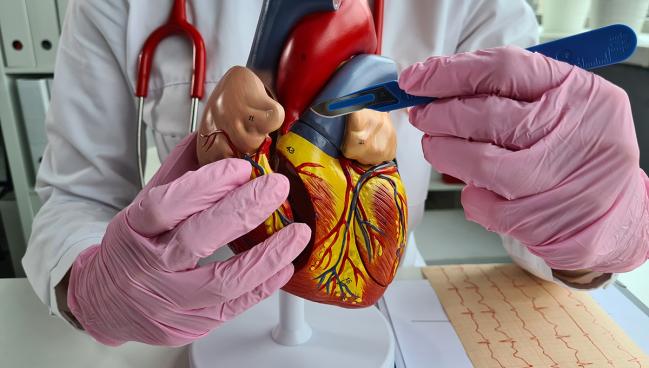
Using a previously “instrumented” radial artery—one that has been used for transradial PCI or invasive angiography—as a bypass graft for cardiac surgery is not associated with poor clinical outcomes or impaired graft patency, new research suggests.
At 2 years, the rate of major adverse cardiac events following CABG with a previously instrumented radial artery was not significantly different when compared with cases where surgeons used a noninstrumented radial artery. The patency of the grafts at just beyond 4 years was also comparable to radial grafts not used in prior cardiac catheterizations.
Garry Hamilton, MBBS (Austin Health, Heidelberg, Australia), who led the study, published this week in Circulation: Cardiovascular Interventions, believes that prior transradial access shouldn’t necessarily preclude the use of a radial artery conduit.
“Although I don't think every patient should have their instrumented radial artery graft used as a conduit, the current dogma to consider prior [transradial access] an absolute contraindication to the use of the radial artery needs reconsideration,” he told TCTMD. “Provided the vessel is structurally intact and not occluded, the instrumented radial artery may be appropriate for use.”
The US coronary revascularization guidelines recommend that after the internal mammary artery is used to bypass the left anterior descending (LAD) artery, surgeons should use a radial artery instead of a saphenous vein to graft the second most important non-LAD vessel. It’s also recommended that surgeons avoid using the radial artery if there has been a prior transradial catheterization.
There is a “long-standing apprehension” about using an instrumented radial artery as a bypass graft, Hamilton explained. This is likely because of known injury that occurs at the time of transradial access and the uncertainty around the extent and timeline of recovery, he said. Additionally, two small observational studies suggested that instrumented radial artery grafts might have worse patency compared with a noninstrumented conduit.
Hamilton said that damage to a radial artery is quite heterogeneous, ranging from “little-to-no damage right through to significant disruption of the vessel architecture and radial-artery occlusion.” In their region, surgeons prioritize arterial grafting where possible, and the instrumented radial artery is often the best conduit available in select patients, he said.
Mario Gaudino, MD, PhD (NewYork-Presbyterian/Weill Cornell Medicine, New York, NY), an author of the US revascularization guidelines, isn’t convinced the new study will shift the current recommendations, however.
“There is lot of evidence that after transradial catheterization, there is damage to the endothelium, even partially to the media, of the radial artery,” he told TCTMD. “There is reduced function in terms of endothelial-mediated vasodilatation, and it’s unclear when function is recovered.”
While interventional cardiology has improved significantly over the years, with the use of less-stiff wires less likely to cause trauma, Gaudino doesn’t think the evidence is sufficient to stray from using the noninstrumented radial artery.
“I’m sure there are selected patients where the surgeon feels comfortable for different reasons to use the radial artery and it is probably okay to use,” he said. “Those are very selected cases, but in the majority of patients, I still believe that the radial should not be used after transradial catheterization. This study does not change that in my opinion.”
Patency and Attrition
The new study included 294 consecutive patients who underwent CABG surgery using both the left and right radial artery. In the noninstrumented group (n = 168), neither radial artery had previously been used to perform PCI or invasive coronary angiography, while the instrumented group (n = 126) included those with at least one radial artery graft subjected to prior transradial access. For CABG, the conduit was selected based on availability and quality, as well as patient demographics and anatomy, and the instrumented radial artery was used as the third most important coronary target following left internal thoracic artery and the contralateral radial artery grafts.
In terms of MACE, a composite of all-cause mortality, MI, or repeat coronary revascularization, the rates were 2.4% and 5.4% in the noninstrumented and instrumented groups at 2 years, a nonsignificant difference (HR 0.44; 95% CI 0.12-1.61).
For 50 patients enrolled in the patency analysis assessed with CT angiography, which occurred a median of 4.3 years after CABG, there was no significant difference between noninstrumented and instrumented grafts (82% vs 80%; P > 0.99). Complete graft occlusion was the most common reason for graft failure, with rates of 16% and 12% in the two groups, respectively (P = 0.77).
Hamilton underscored that follow-up is relatively brief, particularly since many CABG patients would be expected to live years beyond the surgery. Long-term data out beyond 10 years are necessary, but it will be some time before they are available.
“Importantly, we don't know the graft attrition rate of instrumented radial-artery grafts, and that's a focus of ongoing research,” he said. “It’s possible that the damage inflicted during transradial access, regardless of how significant or reversible it is, will result in negative remodeling over time and thus affect patency.”
Hamilton pointed out that other conduits, particularly saphenous vein grafts, also have documented rates of attrition. It’s possible the instrumented radial artery—even if it has inferior long-term patency compared with a noninstrumented artery—might still be the “next best conduit” after the left internal mammary artery to LAD and contralateral radial-artery grafting to the second most important coronary target.
It would be ideal if noninvasive assessments could determine if an instrumented radial artery is viable prior to harvesting. At Hamilton’s center, an instrumented radial artery is considered if it’s palpable, the Allen test is negative, a bilateral ultrasound shows normal velocities, and “it appears relatively normal in comparison to the contralateral radial artery,” he said. Surgeons also visually inspect the artery, discarding those they deem unsuitable.
Cardiac surgeon Marc Ruel, MD, PhD (University of Ottawa Heart Institute, Canada), emphasized that this is a study of bilateral radial CABG in whom some patients had one previously instrumented radial artery. The use of bilateral radial arteries for CABG is quite rare outside of highly select centers, he said.
While Ruel is a proponent of multiarterial grafting, he said there is no evidence suggesting that bilateral radial arteries should be used, or even that a third arterial graft is better than two.
“So, I think we have to think of biology here,” he told TCTMD. “You have a full-thickness perforation in your blood vessel followed by compression, and it’s kind of difficult to predict whether this hole is going to heal. Will it heal by thrombotic occlusion, with stenotic patency from fibrosis or neointimal proliferation, or with normal luminal size? Even if the luminal diameter is normal, will there be perfect endothelial function all along the vessel?”
Ruel said the study is interesting, and while it provides one point of view, his “overall recommendation would be to continue to exert caution in using previously instrumented radial arteries.” Surgeons, he said, should instead focus on providing at least two arterial grafts to CABG patients expected to live a long time.
Lifetime Management Also Key
In an accompanying editorial, Louai Razzouk, MD (New York University Grossman School of Medicine, New York, NY), says that the new study should be considered “hypothesis-generating” at this stage, and that future research may consider standardizing instrumented radial artery graft selection, as well as the use of vasodilators, both periprocedurally and over the long term. Like the researchers, though, Razzouk says the current study suggests that prior transradial access “shouldn’t be considered an absolute contraindication.”
In addition to the relatively short follow-up, Gaudino pointed out that the study is small, making any comparison between groups underpowered for clinical outcomes. The same applies to the patency analysis. Moreover, as an observational study, its patients are highly selected, and there’s no information on how many radial arteries were judged unsuitable by the surgeon.
“I think what this study may potentially tell us is that when the surgeon thinks that the radial artery can be used, the radial artery probably can be used, but that doesn't really mean that the radial artery can be used in every patient after transradial catheterization,” he said.
In terms of practicalities, Gaudino noted that with the way the operating room is set up, harvesting the left radial artery can be performed simultaneously with the left internal thoracic artery, making the surgery more efficient. The cath lab, on the other hand, is set up so that access to the heart is usually through the right radial artery.
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Hamilton GW, Theuerle J, Chye D, et al. Graft patency and clinical outcomes in patients with radial artery grafts previously instrumented for cardiac catheterization. Circ Cardiovasc Interv. 2024;17:e013739.
Razzouk L. The beaten path: use of the radial artery as a bypass graft after instrumentation. Circ Cardiovasc Interv. 2024;17:e014194.
Disclosures
- Hamilton and Razzouk report no relevant conflicts of interest.




Comments