Subcutaneous Furosemide Enables HF Patients to Address Congestion at Home
The AT HOME-HF pilot study suggests this approach to diuretic delivery can safely improve symptoms and function.
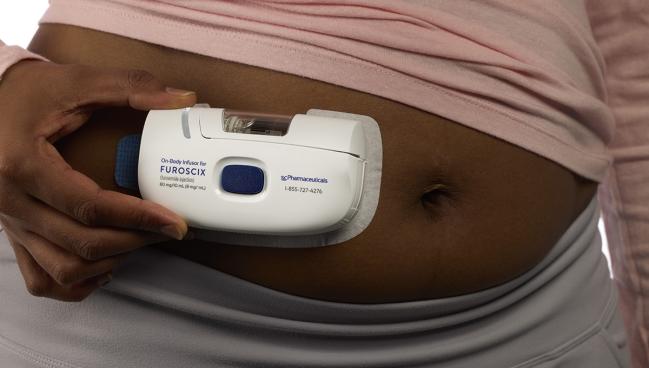
Photo Credit: Adapted from scPharmaceuticals
Yet the study did not meet its primary efficacy endpoint assessing CV death, HF events, and change in N-terminal pro–B-type natriuretic peptide (NT-proBNP), researchers report in a paper published last week in JACC: Heart Failure.
With this type of drug delivery, “the idea is to be able to have self-management of heart failure with worsening congestion without the requirement for hospitalization,” Marvin A. Konstam, MD (Tufts Medical Center, Boston, MA), told TCTMD, adding that the most common reason patients are admitted for HF is to receive diuresis during their hospital stay.
The approach could spare patients a trip to the hospital and save money, he pointed out: over a million HF hospitalizations occur each year in the United States, with a cost that’s in the range of $40 billion and expected to grow.
For the phase II AT HOME-HF study, Konstam and colleagues tested the form of SC furosemide that’s now sold under the brand name Furoscix (scPharmaceuticals) after being approved 2 years ago by the US Food and Drug Administration. Its “pH-adjusted formulation has the same pharmacokinetics and pharmacodynamics” as the IV form of furosemide that’s administered in a hospital setting, said Konstam.
Furoscix is not the only such tool being developed to meet this clinical need, though it is the first to hit the US market. Nor is AT HOME-HF likely the final say for this particular product.
Robert J. Mentz, MD (Duke University School of Medicine, Durham, NC), editor-in-chief of the Journal of Cardiac Failure, told TCTMD he does see a role for SC furosemide in addressing congestion. “It happens every single day that patients then call our office and say, ‘I've been on this pill diuretic, and it's not working. I'm short of breath, what should I do?’ And historically, what we've done is we, most commonly, double up on their loop diuretic,” Mentz noted.
“We try to manage it with pills and oral diuretics, and sometimes . . . it doesn't work. If it continues to worsen, our options are pretty limited,” he continued, so patients end up at an outpatient clinic for IV diuretics or go to the emergency room, with many admitted to the hospital.
AT HOME-HF helps reassure that SC furosemide is safe, added Mentz, who said further research on the topic is justified. The approach could potentially save healthcare resources and costs as well as benefit patients, he said, if it proves able to decrease the number or lengths of hospital stays.
AT HOME-HF
The AT HOME-HF investigators enrolled 51 patients with chronic HF and worsening congestion, randomizing 34 patients to receive SC furosemide with the Furoscix device and 17 to receive usual care. Seven in 10 participants were male, and 84.3% had NYHA functional class III HF.
All patients in the SC furosemide arm received at least one dose of the drug, with a total of 118 doses administered over the 30-day study period and a mean of four doses per person. Most (90.6%) were given within the first 7 days, during which time most patients either had their baseline diuretic regimen maintained or their dosage decreased. In the usual-care group, the most common diuretic strategy was an increase in oral loop diuretic dose, seen in 58.8% of participants; less-used strategies included changing from one loop diuretic to another, adding metolazone, and increasing spironolactone dose.
The primary endpoint—a 30-day hierarchical composite of cardiovascular death, HF events, and change in NT-proBNP—did not significantly favor SC furosemide over usual care (win ratio 1.11; 95% CI 0.48-2.50). However, the furosemide-treated patients had, on average, a 2.02-kg greater reduction in body weight at day 3 (when 69% of furosemide dosing had occurred) as well as significantly greater improvements in 7-point dyspnea score (P = 0.017) and 6-minute walk test (P= 0.032). There also was a trend toward improvement in Kansas City Cardiomyopathy Questionnaire-12 overall summary score favoring SC furosemide (P = 0.106).
About two-thirds of the SC furosemide group and half of the usual-care group had at least one adverse event. Most common were hypokalemia (20.6% vs 0%), renal impairment (14.7% vs 17.6%), infusion-site pain (14.7% vs 0%), and fatigue (2.9% vs 11.8%). Approximately half of the adverse events in SC furosemide patients were related to the study treatment, most often infusion-site pain. One serious adverse event deemed possibly related to SC furosemide occurred: dehydration in a patient who presented to the emergency department 1 week after the last dose with nonbloody diarrhea, nausea, and epigastric pain.
Lessons Learned
While the pilot study didn’t meet its primary endpoint, it did provide some lessons, said Konstam. “We learned that patients are totally able to administer the dose subcutaneously in the abdomen on their own. We learned that it's safe because, really the only adverse effect of consequence was skin irritation at the site, which occurred in a few people.”
Mentz, for his part, praised the study for its inclusion of patients like those he takes care of in practice. Black individuals, for instance, made up 30% of the cohort, and the mean body mass index (BMI) was around 36 kg/m2. “One of the things that we had worried about was if you’ve got more abdominal obesity, and you're putting this sub-Q device on: those individuals that have a larger BMI, . . . will they get adequate diuresis?” he observed, so it’s good to see how it performed in a realistic cohort.
One possible downside, though, is that patients must be closely monitored: in this study, they had follow-up (by phone or in clinic) each day for the first week. “That’s pretty resource-intensive,” said Mentz. Even with this monitoring, or perhaps because of this extra awareness, there was more hypokalemia in the SC furosemide group, he noted.
Larger studies are needed to show not only that the treatment is effective but also that it’s safe in wider use, Mentz said. These could also help develop a “framework for how to do this in a way that it can be expanded to practices that may have less experience or less support or infrastructure.”
Konstam said there are ongoing discussions about what research to pursue next. Many of the endpoints assessed by AT HOME-HF, like function and quality of life, are meaningful for patients, said Konstam, though “ultimately, we'd love to show that it does reduce overall hospitalizations.” Also, there are details to be worked out in terms of monitoring patients and the optimal length of treatment, he specified.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Konstam MA, Massaro J, Dhingra R, et al. Avoiding treatment in hospital with subcutaneous furosemide for worsening heart failure: a pilot study (AT HOME-HF). JACC Heart Fail. 2024;Epub ahead of print.
Disclosures
- This study was funded by scPharmaceuticals.
- Konstam reports having received personal fees from Alnylam, Boehringer Ingelheim, Cardurian, LivaNova, scPharmaceuticals, Fire 1, Vicardia, and Alleviant.





Comments