Two Trials Boost Intra-arterial Lytics After Stroke Thrombectomy
The results increase “my confidence that this approach has merit,” Lauren Sansing says. More trials are on the way.

Just a month after trial results appeared to dampen prospects for the use of intra-arterial thrombolysis on top of stroke thrombectomy in patients with large-vessel occlusions (LVOs), two new trials have pointed to significant benefits with the approach.
In ANGEL-TNK and PEARL, intra-arterial administration of tenecteplase and alteplase, respectively, after successful thrombectomy increased the proportion of patients with an excellent neurological outcome at 90 days compared with standard medical treatment. Rates of symptomatic intracranial hemorrhage and mortality were not significantly different between groups.
The results of both trials, which were reported last week at the International Stroke Conference (ISC) in Los Angeles, CA, contrast with those from the POST-UK and POST-TNK trials released last month, which showed no significant impact on 90-day outcomes with intra-arterial urokinase or tenecteplase. The two new studies are consistent with the older CHOICE trial, which demonstrated improved outcomes with intra-arterial alteplase.
Although the trial evidence is mixed, with these two latest trials being positive, “I think it’s pushing my confidence that this approach has merit,” Lauren Sansing, MD (Yale School of Medicine, New Haven, CT), chair of ISC 2025, told TCTMD.
Despite thrombectomy’s success at improving functional outcomes among patients with acute ischemic strokes caused by LVOs, many patients continue to have poor outcomes despite recanalization, which could be due to additional clots in the more-distal vessels or unseen clots in the microcirculation causing a no-reflow phenomenon.
That is a real problem for patients, Sansing said.
“We get the proximal vessel open in the overwhelming majority of patients and yet there’s not this distal perfusion that’s always restored,” she said. “So, this idea of putting low-dose thrombolytic into it so that you can hopefully restore blood flow to the smaller blood vessels has biological merit. . . . And I think we’re seeing the scales sort of move towards [indicating] that indeed, with either alteplase or tenecteplase, this does work.”
Although CHOICE supported that idea, interpretation of its results was muddied by the premature stoppage of the study, which resulted in a small sample size.
ANGEL-TNK
ANGEL-TNK, conducted at 19 sites in China, was one of the trials designed to replicate the CHOICE results. Investigators enrolled 255 patients (median age 72 years; 45% women) who had an ischemic stroke caused by an LVO in the anterior circulation, presented from 4.5 to 24 hours after they were last known to be well, and underwent successful thrombectomy resulting in an eTICI 2b50-3 score (at least 50% reperfusion). Patients who received IV thrombolytics before the procedure were excluded, and IV antiplatelet agents and heparin were not allowed during the procedure.
The investigators randomized patients to an intra-arterial infusion of tenecteplase 0.125 mg/kg (maximum 12.5 mg) administered over a period of more than 15 minutes after thrombectomy or to standard medical treatment without intra-arterial lytics.
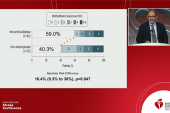
The primary endpoint was the proportion of patients who had an excellent neurological outcome—modified Rankin Scale (mRS) score 0-1—at 90 days. As reported by Xiaochuan Huo, MD, PhD (Beijing Anzhen Hospital, China), patients treated with intra-arterial tenecteplase were more likely to achieve this benchmark (40.5% vs 26.4%; P = 0.02).
In terms of safety, there were no differences between the treatment and control arms in symptomatic intracranial hemorrhage within 48 hours (5.6% vs 6.2%), any intracranial hemorrhage within 48 hours (24.6% vs 27.9%), or death at 90 days (21.4% vs 21.7%; P = NS for all).
PEARL
PEARL, presented by Raul Nogueira, MD (University of Pittsburgh, PA), and Yamei Tang, MD (Sun Yat-Sen Memorial Hospital, Guangzhou, China), was a similar trial conducted at 28 stroke centers in China.
Investigators enrolled 324 patients (mean age 66 years; 31% women) who had an LVO stroke in the anterior circulation, presented within 24 hours of when they were last known to be well, and had successful thrombectomy resulting in an eTICI 2b50-3 score. The patients were randomized to receive an intra-arterial infusion of alteplase 0.225 mg/kg (maximum 20 mg) or standard medical treatment without intra-arterial lytics. In contrast to ANGEL-TNK, 42% of patients received IV thrombolysis before thrombectomy.
The primary endpoint in PEARL also was an mRS score of 0-1 at 90 days, with a higher proportion reaching that goal after receiving intra-arterial alteplase (44.8% vs 30.2%; RR 1.45; 95% CI 1.08-1.96).
There were no significant differences between the treatment and control groups in symptomatic intracranial hemorrhage within 36 hours (4.3% vs 5.0%), any intracranial hemorrhage within 36 hours (32.9% vs 26.9%), or all-cause mortality at 90 days (17.1% vs 11.3%; P = NS for all).
Making Sense of the Mixed Results
At an earlier session during ISC 2025, Thanh Nguyen, MD (Boston Medical Center, MA), presented a pooled analysis of the POST-UK and POST-TNK trials. With the increased statistical power from the combined cohort, there was a significant benefit of intra-arterial thrombolysis—the proportion of patients with an mRS score of 0-1 at 90 days was 47.1% with intra-arterial treatment and 42.2% without it (P = 0.045).
Speaking with TCTMD, Nguyen said ANGEL-TNK and PEARL might have been positive despite having smaller samples sizes than POST-UK and POST-TNK due to the inclusion of a broader range of patients. In the newer trials, most patients had eTICI 2b scores after thrombectomy, signaling less complete reperfusion compared with the patients in the POST trials, which included only those with eTICI scores of 2c or better after the procedure.
“It’s possible that incomplete reperfusion is what’s driving the outcomes for the [intra-arterial] lytics, although we do see a signal for those who have near-complete or complete reperfusion as well,” Nguyen said.
In addition to differences in the patient population, Huo also pointed to the fact that IV thrombolysis wasn’t allowed before thrombectomy and that IV antiplatelets and heparin weren’t allowed during the procedure as a potential explanation for the better outcomes in ANGEL-TNK compared with the POST trials. Tenecteplase was administered slowly and in a fixed position to avoid hemorrhagic transformation, he added.
Although there are now multiple positive trials of intra-arterial lytics, Nguyen pointed to the need for additional data from ongoing studies that will be reporting results relatively soon before determining whether this is the right approach. “We will need to digest the collective data and take a look once we have all the information.”
If this strategy is adopted, “it changes the paradigm of care,” she noted. “This is not something that we were routinely thinking about a few years ago. The question is, does it really improve outcomes?”
These ongoing trials are testing whether the approach boosts the proportion of patients with an excellent functional outcome (mRS score 0-1), rather than a good outcome with perhaps more symptoms. The excellent result is “a meaningful outcome, but what if you have a symptomatic hemorrhage? You could lose what you’ve gained,” Nguyen said. “The data doesn’t support an increase in symptomatic hemorrhage, which is good, but this is something to think about.”
Sansing pointed out, too, that the no-reflow phenomenon is not always caused by additional clot that would be amenable to treatment with intra-arterial lytics.
“We’re headed towards, I think, the evidence showing that the thrombolytic does have benefit,” she said, adding, however, “I think there’s a lot more to learn about the biology there.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Huo X. Intra-arterial tenecteplase thrombolysis after successful endovascular therapy. Presented at: ISC 2025. February 7, 2025. Los Angeles, CA.
Tang Y, Nogueira RG. Intra-arterial alteplase for acute ischemic stroke after mechanical thrombectomy (PEARL): a multicenter randomized trial. Presented at: ISC 2025. February 7, 2025. Los Angeles, CA.
Nguyen TN. Efficacy and safety of intra-arterial thrombolysis following endovascular reperfusion for large-vessel occlusion stroke: an individual patient level pooled analysis of POST-UK and POST-TNK randomized trials. Presented at: ISC 2025. February 6, 2025. Los Angeles, CA.
Disclosures
- ANGEL-TNK was funded by unrestricted grants from China Shijiazhuang Pharmaceutical Company Recomgen Pharmaceutical (Guangzhou) Co., Ltd.
- Huo reports no relevant conflicts of interest.
- PEARL was funded by unrestricted grants from the Sun Yat-Sen Memorial Hospital Clinical Research 5010 Program and Genesis MedTech.



Comments