AHA Updates Its Scientific Statement on CIED Infections
The field has seen advances in prevention, diagnosis, and management, but randomized data are still mostly lacking.
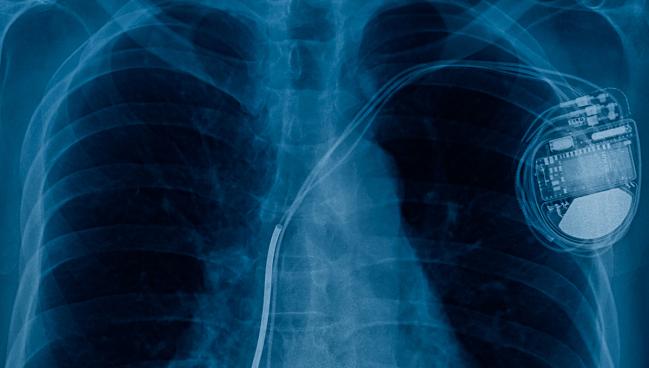
The American Heart Association (AHA) has provided an update to its scientific statement on cardiovascular implantable electronic device (CIED) infections, reflecting advances in the field since the first such document was issued back in 2010.
A lot has happened since then in the areas of prevention, diagnosis, and management, Larry Baddour, MD (Mayo Clinic, Rochester, MN), chair of the update’s writing group, told TCTMD.
He and vice chair Daniel DeSimone, MD (Mayo Clinic), highlighted randomized trials focused on prevention, evolving knowledge on the utility of advanced imaging for diagnosis, and the emergence of newer devices—including leadless pacemakers and subcutaneous implantable cardioverter-defibrillators (S-ICDs)—that are expected to carry lower risks of infection.
A key point is that “we want to keep devices that are not infected in the patient’s body. If it’s infected, we want to take it out,” DeSimone noted to TCTMD, “It’s not one part that’s taken out, it’s the whole device, the entire system. And that comes with risk to the patient, morbidity as well as mortality. In addition to that, [there’s] significant financial cost to the healthcare system.”
Patients who develop CIED infections can be difficult to manage, and a team approach incorporating clinicians with a broad range of expertise—including electrophysiology, infectious diseases, cardiovascular surgery, nuclear medicine, echocardiography, and others—is critical, Baddour underscored. “These patients are usually older and have multiple comorbid conditions and deserve multispecialty care in larger-volume medical centers.”
Gains in Prevention and Diagnosis
The updated statement, published online last week in Circulation and endorsed by the International Society for Cardiovascular Infectious Diseases, covers three main areas: prevention, diagnosis, and management.
For prevention, Baddour noted that there have been two randomized trials conducted since 2010. PADIT evaluated compared incremental periprocedural antibiotics with conventional treatment in patients receiving CIEDs, with no difference in infection rates observed. In WRAP-IT, a novel antibiotic-impregnated envelope used at the time of device placement in conjunction with standard prevention strategies reduced major infections by a relative 40%. The envelope, the authors say, may be considered in patients considered to be at high risk for CIED infection, including those who are at risk for perioperative hematoma formation.
We want to keep devices that are not infected in the patient’s body. If it’s infected, we want to take it out. Daniel DeSimone
In the area of diagnosis, the paper includes discussion of the challenges of distinguishing between infected and noninfected devices, the use of various imaging modalities, laboratory advances in pathogen detection, and the role of inflammatory markers.
As the update makes clear, questions have been raised about the use of transesophageal echocardiography (TEE) to identify infection in a patient with a CIED, and in recent years, there has been growing use of positron emission tomography/computed tomography (PET/CT) imaging to aid in diagnosis. Echocardiography is still used but combining it with PET/CT has increased the sensitivity and specificity of diagnosis, DeSimone said.
Ruling out an infection is “easier said than done, and these tests, even combined, are not 100% accurate,” he added.
An important aspect of CIED infections for cardiologists to understand, DeSimone noted, is “that there is a spectrum of organisms that are more likely to cause infection and stick to these devices and certain ones that are not, which will impact the pretest probability of our diagnostic imaging.”
Management and Other Issues
Once the diagnosis of CIED infection is made, the entire system should be removed whenever possible, stresses the management section of the statement. The authors then address the optimal timing of reimplantation or implantation of a newer device, the role of percutaneous mechanical aspiration for vegetation removal, and strategies for treating patients in whom an infected CIED cannot be removed due to major comorbidities, a high extraction risk, or limited life expectancy.
Percutaneous mechanical aspiration has been used in select patients, demonstrating some benefit in, for example, reducing the risk of pulmonary embolism at the time of device extraction, Baddour said. In addition, the document discusses a strategy of administering antimicrobials directly into an infected pocket.
Other noteworthy issues covered by the document include the emergence of newer devices that might help mitigate the problem of CIED infection. Leadless pacemakers and S-ICDs have been evaluated in both prevention- and management-related studies.
There are also several risk scores that can help estimate the risk of CIED infection, and these “may be useful in identifying potential candidates for incremental prevention measures, and alternative device platforms such as a leadless pacemaker or S-ICD,” Baddour et al write.
A Call for Randomized Data
The document was designed to help clinicians navigate some common clinical scenarios, and most of the sections are introduced in the form of questions that are frequently asked in daily practice. The authors include diagnostic and management algorithms for suspected CIED pocket infections and suspected CIED lead/valvular infection without pocket infection.
But despite the advances that have been made in CIED infection, much more randomized data are needed to provide guidance to practicing clinicians, Baddour said, pointing out that there are no RCTs in the areas of diagnosis and management. The dearth of randomized data and the dependence on consensus opinion has led to some differences across various guidelines that have addressed CIED infections over the years.
“Prospective, randomized controlled trials are needed to enhance the diagnosis and management of CIED infection,” Baddour stressed. “We want to operate on data from such trials as we think about supporting our practice, supporting what we do.”
Despite the need for more-robust evidence to guide practice, the aim of the current statement is to ease decision-making, with the supplemental material containing six case scenarios that lay out potential solutions to common clinical conundrums.
Regarding the document, DeSimone said, “Hopefully it’s valuable for front-line clinicians as they encounter some of these difficult scenarios.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Baddour LM, Garrigos ZE, Sohail MR, et al. Update on cardiovascular implantable electronic device infections and their prevention, diagnosis, and management: a scientific statement from the American Heart Association. Circulation. 2023;Epub ahead of print.
Disclosures
- Baddour and DeSimone report no relevant conflicts of interest.



Comments