ALIGN-AR: Positive Results for Trilogy Valve in Aortic Regurgitation
If the device is approved, “I am very excited to put this to use because we have an unmet need,” Pinak Shah says.
SAN FRANCISCO, CA—The Trilogy heart valve system (JenaValve) performs well in patients who have symptomatic aortic regurgitation (AR) and a high surgical risk, according to the results of the ALIGN-AR trial.
The single-arm trial met both safety and efficacy goals established based on historical data, with significant gains observed in NYHA functional class and quality of life through 1 year that were accompanied by “excellent” echocardiographic outcomes, including improvements in various measures of LV remodeling, Vinod Thourani, MD (Piedmont Heart Institute, Atlanta, GA), reported Tuesday at TCT 2023.
Though the overall pacemaker implantation rate overall was 24%, it declined as operators gained experience with the procedure, reaching 14% in the last third of patients enrolled in the trial.
“The TRILOGY THV system provides the first dedicated TAVR option for symptomatic patients with moderate-to-severe or severe aortic regurgitation who are at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population,” Thourani said during his presentation.
When untreated, severe symptomatic AR is tied to a high rate of mortality, especially in patients with NYHA III or IV symptoms. SAVR is the only recommended therapy for patients with severe AR, although many high-risk patients are not offered surgery. When TAVI devices are used off-label in this population, there are high rates of complications, including paravalvular regurgitation and embolization, Thourani noted.
The Trilogy system is specifically designed for patients with AR, with three locators that go into the aortic sinuses and, after alignment, anchor onto the leaflets; the valve is then deployed. This implantation technique protects against device embolization. If the Trilogy is ultimately approved by regulators, it will provided a much-needed therapeutic option, experts indicated. The ALIGN-AR trial was designed as a US Food and Drug Administration investigational device exemption trial.
Pinak Shah, MD (Brigham and Women’s Hospital, Boston, MA), commenting at a press conference, predicted that uptake would be swift after an approval, downplaying the pacemaker rate because of the lack of alternatives for these patients. “It’s a little bit higher than what we see in normal TAVR, but I am very excited to put this to use because we have an unmet need,” he said.
ALIGN-AR
The ALIGN-AR trial, conducted at 20 sites, included 180 patients (mean age 75.5 years; 47.2% women) who had symptomatic moderate-to-severe or severe AR (grade ≥ 3), NYHA class II-IV symptoms, and a high risk for surgery as determined by a heart team evaluation. Mean STS score was 4.1%, and one-third of patients had frailty. AR was graded as severe in 64.4%.
General anesthesia was used in 91.1% of procedures, which lasted a mean of 71.8 minutes. Rates of technical, device, and procedure success exceeded 92%. There were no cases of intraprocedural death, annular rupture, ventricular perforation, or coronary obstruction, with low rates of valve embolization (2.2%) and aortic dissection (0.6%).
Primary safety and efficacy endpoints were based on prespecified performance goals established using historical data.
The safety endpoint was a composite of 30-day all-cause mortality, any stroke, major vascular complication, life-threatening/major bleeding, new pacemaker, acute kidney injury, valve dysfunction, and surgery/intervention related to the device. The observed rate was 26.7%, and the upper end of the confidence interval was 34.1%, significantly below the prespecified 40.5% margin for noninferiority (P < 0.001).
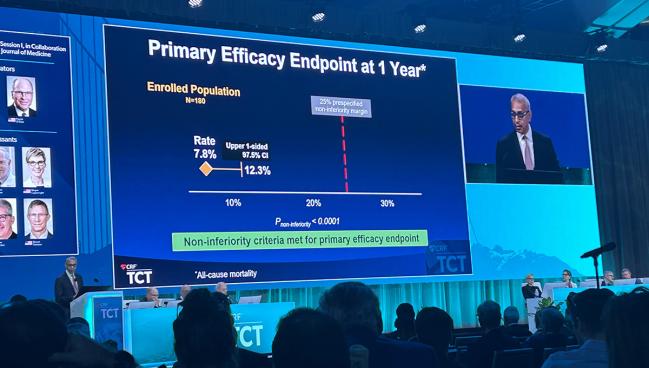
The efficacy endpoint was all-cause mortality measured at 1 year. The observed rate was 7.8%, and the upper end of the confidence interval was 12.3%, significantly below the performance goal of 25% (P < 0.0001 for noninferiority).
Safety events were dominated by new pacemaker implants, which declined as the study progressed. Thourani attributed that to changes that were made to insertion technique, less aggressive oversizing of the valve, and advances in the management of periprocedural conduction abnormalities.
He highlighted the angiographic results, which showed that more than 99% of patients had none/trace or mild paravalvular regurgitation at 30 days, with that performance sustained throughout follow-up. At 1 year, in fact, no patients had regurgitation graded as moderate or worse.
Through 1 year, patients saw significant improvements in LV end-systolic diameter, LV end-systolic volume, LV mass, and LV mass index (P < 0.0001 for all). “You see really significant improvements in the ventricle after having the aortic regurgitation obliterated,” Thourani said.
In addition, the effective orifice area was large (mean 2.8 cm2 at 1 year), with a significant decline in mean gradient observed from baseline to 1 year (8.7 to 4.3 mm Hg).
Accompanying those changes were improvements in NYHA functional class (all had class II or worse symptoms at baseline versus 40% at 1 year) and Kansas City Cardiomyopathy Questionnaire overall score, which increased from 55.8 at baseline to 77.6 at 1 year.
A Forgotten Disease?
At the TCT press conference, Thourani said the potential population that could be treated by the Trilogy is huge, adding that AR is “a forgotten disease.” The number of patients presenting for inclusion in the continued access phase of this trial is greater than the number that were getting enrolled in the initial phase because of increasing awareness that there’s now a possible treatment, he indicated. “Once we open this up, I think we’ll see this proliferation of a disease managed that we normally have ignored.”
Other experts also were encouraged by the findings.
“You’re talking about a high-risk patient population that didn’t have surgical options, and what you’re seeing here is very excellent results,” Rebecca Hahn, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), told the media.
Bernard Prendergast, MD (St Thomas’ Hospital, London, England), highlighted the differences between patients with AR and those with aortic stenosis, noting that anatomy and rates of aortopathy are distinct. “Therefore, we need a different device. So using currently available TAVR devices off-label to treat aortic regurgitation can be successful, but it is fraught with risk of embolism and other adverse consequences,” he said. With the Trilogy, he added, “we now have a very useable device which is specific for aortic regurgitation, and although it’s a less common disease than aortic stenosis, it’s one with major need. So I’m really excited by these results.”
Robert Bonow, MD (Northwestern University Feinberg School of Medicine, Chicago, IL), serving as a discussant after Thourani’s presentation, found the data encouraging, as well, but questioned Thourani about whether there is an RCT in the works to pit the Trilogy valve against a comparator in patients with AR. “You could argue unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for these patients,” Bonow said, pointing, for example, to the four pillars of heart failure therapy.
Thourani said a randomized trial in high-risk patients is unlikely at this point, but added that there is a lot of interest in exploring the impact of Trilogy in patients with intermediate or low risk and that the ALIGN-AR investigators have mulled an all-comers trial.
“If we go into younger patients . . . a randomized trial is warranted,” Thourani said.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Thourani VH, Vahl TP. The JenaValve Trilogy heart valve system in high surgical risk patients with symptomatic, severe aortic regurgitation: the ALIGN AR trial. Presented at: TCT 2023. October 24, 2023. San Francisco, CA.
Disclosures
- Thourani reports grant support/research contracts from Abbott, Edwards Lifesciences, and Boston Scientific and consulting fees/honoraria/speaking fees from DASI Simulations.
- Bonow reports no relevant conflicts of interest.






Comments