JenaValve Trilogy Meets the Challenge of AR in Early Real-World Use
Results with the TAVI device, the first approved for use in aortic regurgitation, now need to be replicated on a larger scale.
PARIS, France—New results from Europe provide reassurance that TAVI with the Trilogy heart valve system (JenaValve Technology) can be safe and effective patients with aortic regurgitation (AR), even outside of a trial setting.
Just a year ago, Trilogy received CE Mark approval for use not only in AR but also in aortic stenosis, making it the one device in the world approved for both indications. And, as TCTMD reported last fall, initial results were positive. It has yet to get Food and Drug Administration clearance for marketing in the United States, where the ALIGN-AR pivotal trial is currently recruiting participants.
Today, in a hotline session at EuroPCR 2022, Alexander R. Tamm, MD (Johannes Gutenberg-Universität, Mainz, Germany), presented data on 45 consecutive patients at high surgical risk treated at six German centers.
Speaking with the media, Tamm noted that until recently, TAVI in AR was done off-label using devices designed for aortic stenosis. This unique disease subset has posed “procedural challenges and poorer outcomes,” said Tamm.
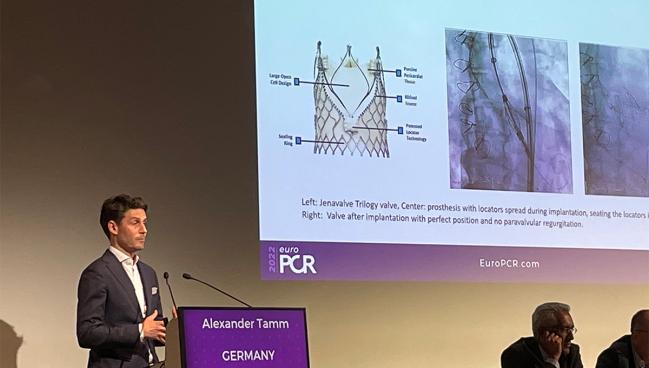
Aortic valve calcification, which plays a key role in anchoring most TAVI devices, tends to be absent or mild in most cases. Trilogy, to meet this need, “features unique locators that [allow] the valve to ‘clip’ onto the native leaflets,” he explained. “Thus, it’s enabling anchoring in pure AR patients with noncalcified valves.”
Tamm told TCTMD that “the technique is the game changer.” With Trilogy, three locators clip to the aortic leaflets and then the stent frame clips to the locators with the valve in between, “so you will achieve 100% anchoring,” he said. This allows the device to be fixed and sealed in place once it opens, which protects against valve migration and paravalvular leak.
He described it as an “easy, manageable procedure,” with device times of 20 minutes at the start of the learning curve and 10 minutes with experience.
Commenting on the study for TCTMD, Raj Makkar, MD (Cedars-Sinai Medical Center, Los Angeles, CA), said, “These results are excellent, given that these are the first few implants of these operators in the real world. . . . If the numbers are replicated with a larger sample size, this is for sure, in my opinion, going to be a really important device in treating patients with aortic regurgitation.”
As for the device design, assets include the fact that it’s self-expanding, Makkar said. It also offers “dual anchoring” at the level of the leaflets plus, thanks to oversizing, at the annulus. Moreover, “the hemodynamics are almost best in class,” he noted, adding that “the way this device is placed, there is commissural alignment, so I think access to coronary arteries is going to be good.”
Technical Success, Safety, Effectiveness
In their series, all patients presented with moderate-severe or severe AR. Mean age was 77 years, 40% of patients were female, and the mean EuroSCORE II was 7.1%. Seven in 10 patients were in NYHA class III or IV. Nearly one-third had previously undergone cardiac surgery. More than half (58%) had an LVEF ≤ 50%.
All cases were done via transfemoral access, with the majority (82%) performed under conscious sedation. Two involved postdilatation. Mean procedure time was 77 minutes.
Technical success with a mean gradient < 20 mm Hg and reduction of more than one AR grade, the primary efficacy endpoint, was achieved in 100% of patients. None developed stage 2/3 acute kidney injury. There were no instances of major or life-threatening bleeding, and the minor bleeding rate was 6.7%. One patient (2.2%) had a major vascular complication.
Regarding safety, none of the patients required conversion to open surgery, had a stroke, or died. Nine patients (23%) needed a new pacemaker.
At discharge, mean aortic valve gradient was 4.04 mm Hg and mean aortic valve area was 2.62 cm2. Most patients had no or trace paravalvular regurgitation (56% and 36%, respectively), while 8.9% had mild.
Individuals “ineligible or at high risk for SAVR were previously without treatment options, and our results give confidence to TAVI as a lifesaving procedure for AR patients,” he said, adding that future studies are needed in “larger cohorts and expanded patient populations.”
Although the pacemaker rate might seem to be on the high side, prior studies show this is typical with native AR. A 2020 paper published in Mayo Clinic Proceedings, for example, showed rates of around 20% with TAVI and 12% with surgery, Tamm said. “It’s a different population [from aortic stenosis]. We have to learn about that, I think. Maybe they’re prone to conduction disturbances.”
Makkar drew attention to the pacemaker rate as well, and he predicted this may decrease over time. Moreover, “in a lot of these patients with AR, a pacemaker rate of 18% or 20% is not a bad bargain in terms of avoiding super-high-risk surgery,” he pointed out.
In aortic stenosis, Trilogy may prove useful for certain scenarios, such as when the coronaries are low, said Makkar. With this anatomy, “there is a higher risk of occlusion of the ostia, because the leaflets can be pushed out in front of the coronaries,” he explained.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Tamm AR. JenaValve Trilogy for treatment of aortic regurgitation: worldwide first results. Presented at: EuroPCR 2022. May 18, 2022. Paris, France.
Disclosures
- Tamm reports receiving honoraria or consulting fees from Edwards Lifesciences, JenaValve, and Medtronic.
- Makkar reports serving as a principal investigator for the JenaValve ALIGN-AR pivotal trial.





Comments