My Takeaways From ACC 2018: Everyone Missing From Clinical Trials, Please Stand Up
Are clinical trials in cardiology doing any better at enrolling women? Minorities? Nanette Kass Wenger thinks this is an idea whose time has come.

ORLANDO, FL—If you take away anything from the American College of Cardiology (ACC) 2018 Scientific Session, let it be the lesson that for cardiovascular topics that have been studied to death—think cholesterol pills, antiplatelet combos, new stents, etc—the big leaps of the sort we’ve seen in the past are going to be few and far between.
What’s also clear is that the next most-bang-for-buck strategies are going to need to look at how treatments already proven to work can, in fact, get to the people who need them. On the flip side, the door remains open to the idea that drugs and devices in trials that missed their primary endpoints or produced only modest benefits may yet work, or work better, if tested in the right people.
The most powerful image I’ll hang onto from this year’s conference has nothing to do with ODYSSEY or LIFEVEST, blood pressure polypills or NOAC antidotes. It does have something to do with the “Black Barbershop” study, but I’ll get to that later. For me, the most powerful image of this year’s meeting was Nanette Kass Wenger, MD (Emory University, Atlanta, GA), 88 years young, quoting Victor Hugo in the Simon Dack Keynote lecture: “There is nothing as powerful as an idea whose time has come.”
Wenger’s talk tracked the decades-long journey to today’s understanding of heart disease in women. “As recently as the end of the last century, cardiovascular disease was considered a man’s disease and that’s where the attention was placed,” she said. Even today, women’s symptoms are more often to be dismissed as noncardiac and lifesaving therapies are unequally applied.

Wenger also tackled the underrepresentation of women in research, a topic I’ve been meaning to look into since last year’s European Society of Cardiology (ESC) Congress, where the gender mix in several trials drew my attention. Back in Barcelona, the blockbuster trial was COMPASS. Of the more than 27,000 subjects enrolled, just 23% were women. Tellingly, however, I had to get that number from the published paper—the Hot Line session slides made no mention of the male/female mix in the study. Elsewhere at ESC, I wrote a story about VIVA, an ambitious study that enrolled a whopping 25,000 men (and only men) and demonstrated that population-wide screening for abdominal aortic aneurysm, peripheral artery disease, and hypertension might help reduce all-cause mortality.
VIVA was funded by grants from the European Union and the Danish Council for Independent Research. When I was writing up VIVA, I couldn’t help but ask: why on earth were organizations representing entire populations funding research that would benefit just half?
That’s not so unusual, it turns out. Wenger, in her talk, noted that between 1996 and 2007, women made up just 27% of patients enrolled in US National Institutes of Health (NIH)-funded trials. That’s despite the NIH Revitalization Act of 1993, intended to boost the inclusion of women and minorities in federally funded research. Without setting specific targets, however, the Act’s main achievement seems to have been paving the way for good intentions. An analysis of all NIH-funded randomized trials that were published in 2015 shows 35 of the 142 studies limited enrollment to a single sex and the remainder had a median enrollment of women of 46%. Fully 16 trials enrolled fewer than 30% women, and only 13% of the studies analyzed or reported outcomes by race or ethnicity.
A Window on Women
For the nearly two decades I’ve been writing about advances in cardiology, I’ve been hearing about the underrepresentation of women and minorities in cardiovascular clinical trials. I confess, I’ve also assumed things had likely improved over this period. Spurred in part by Wenger’s talk, I elected to take an informal look.
My analysis is by no means rigorous—go ahead, write and tell me about the trials I’ve left out—but here’s what I did. I looked back at the major international cardiology meetings and journal publications of the last 10 years and pulled what I believe are the three biggest randomized controlled trials from each year. I then looked up the reported percentage of women in each of the trials.
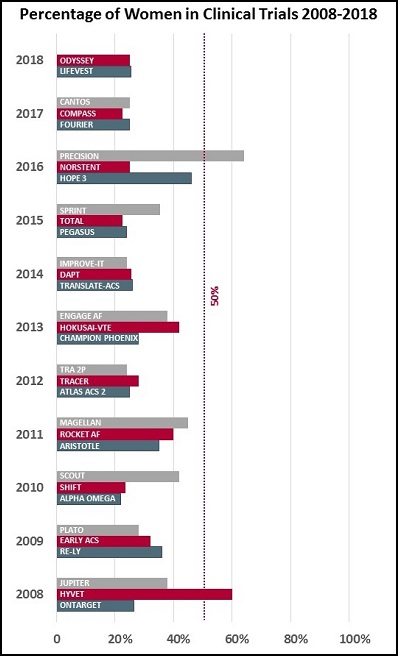
The results depressed me. It turns out that, with a few exceptions, the proportion of women being studied in clinical trials has stayed more or less constant over the last decade. In my analysis, only two trials—PRECISION (looking at the cardiovascular safety of pain medications in patients with osteoarthritis or rheumatoid arthritis) and HYVET (looking at indapamide alone or with perindopril in hypertensive adults over aged 80)—managed to enroll more women than men.
Age is a key issue, Wenger noted in her talk: “The exclusion of elderly patients from clinical trials doubly disadvantages women because of the predominance of coronary events in women at older age.”
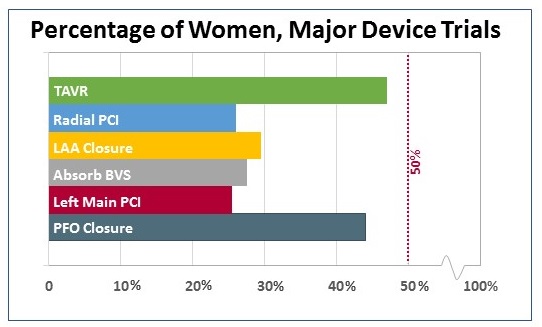
Out of curiosity, I looked separately at major device trials, which tended to be smaller in terms of subjects but have had a major influence on practicing physicians (and their patients). Here, the TAVR trials and the PFO closure trials stand out for enrolling a relatively high proportion of female patients. At least in the case of TAVR, that proportion speaks in part to the number of elderly patients enrolled, particularly in the first high-risk studies.
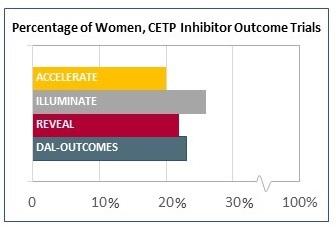
The doomed CETP inhibitor trials I elected to break out separately, since these studies were among the largest of the last decade, representing millions of dollars of industry investment. Yet the agents have ultimately been abandoned—or nearly. A dim flicker of hope for this class of drug currently lies in pharmacogenetic studies hoping to prove that carriers of an ADCY9 single nucleotide polymorphism may benefit from therapy. But here’s a wacky idea: since the average proportion of women enrolled in these enormous trials also hovered in the 23% range, is it a given that these drugs would also have flopped if enrollment had been 77% women? I don’t suppose we’ll be finding out soon.
Of note, I started trying to do a similar analysis of the racial mix in all of these big-name trials and swiftly noticed how inconsistently this information is even reported.
 There are lots of reasons why women aren’t studied in clinical trials, Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai, New York, NY), told me last week. Disease processes may be different in women than in men, women develop disease at a later age, and at least in the case of coronary diseases, they make up just 30% of the people heading to the cath lab for PCI. “So you can’t expect a 50% enrollment of women for studies in a subspecialty where the prevalence for that procedure is different,” she said.
There are lots of reasons why women aren’t studied in clinical trials, Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai, New York, NY), told me last week. Disease processes may be different in women than in men, women develop disease at a later age, and at least in the case of coronary diseases, they make up just 30% of the people heading to the cath lab for PCI. “So you can’t expect a 50% enrollment of women for studies in a subspecialty where the prevalence for that procedure is different,” she said.
The thing is, prevalence of the procedure and prevalence of the disease are not lining up, something Mehran is quick to acknowledge is an entirely separate can of worms. She would like to see more research investigating the reasons why women and minorities are less likely to seek treatments, less likely to end up at the hospitals where clinical research is performed, and less likely to agree to participate in studies.
“This needs to be a multifaceted approach and the answer is not that we can’t do it, because I would say we’ve done very little to make sure [unrepresented groups] are enrolled in our clinical trials, and we can do much, much more,” Mehran stressed. “And the answer is not, oh, they didn’t want to be part of the trial—we need to understand why they didn’t want to come into the trial.”
A number of recent initiatives, most notably PLATINUM-DIVERSITY trial and the WIN-HER initiative, have set out with the specific aim of understanding clinical and nonclinical factors that affect clinical trial enrollment and outcomes.
Wenger touched on similar themes in her keynote, saying she would like to see more research “that addresses beliefs and behaviors, issues of community (local, national, and global), economic issues, environmental issues, and importantly ethical precepts, issues of legislation and policy, public policy, societal and sociocultural issues.”
Indeed, research that embraces sociocultural issues is a good description of the study that many people identified as the most impactful of ACC 2018: the Black Barbershop study. Enrolling only black patrons of 52 barbershops, all of whom had uncontrolled hypertension at baseline, the study—which involved pharmacists meeting up with the men at the barbershops—saw systolic blood pressures fall by an average of 27 mm Hg over 6 months.
“This is something that clinical trialists dream of, that you get this kind of impact in healthcare,” Eileen Handberg, PhD (University of Florida, Gainesville), told the media at a press conference after the presentation. “This is taking care to where patients live, which is sort of a novel approach. This is harnessing the potential of relationships again. This is high-touch medicine.”
The Carrot and the Stick
Designing trials tailored to the swaths of population that have long been underserved will be a key piece of the puzzle. But as Wenger pointed out in her talk, help is also coming from an unexpected source.
In 2016, the US Congress passed the Research for All Act, which mandates adequate representation of women and minorities in all federally funded research. It also requires the Food and Drug Administration (FDA) to mandate appropriate representation, such that pivotal trials intended to secure regulatory approval have sufficient data to determine safety and effectiveness for both women and men, with outcomes analyzed separately for men and women. What’s more, basic research projects must include both male and female cells, tissues, or animals.
“This is essentially game-changing legislation,” Nanette Kass Wenger
“This is essentially game-changing legislation,” Wenger told me in an interview. This means applications being submitted now to the NIH that do not have a plan for enrolling a significant number of women are being turned away. “The same thing has been mandated to the FDA so that as industry comes to the FDA with their planned trials, the FDA will tell them that they must include a representative number of women if their label is ultimately going to identify that this is appropriate for women,” she added.
This may mean blocking enrollment of certain subjects (think: white, middle-aged men) when target enrollment is reached, with consecutive enrollment continuing for underrepresented groups.
“There are ways to do this and there are some major examples of trials that have done this very well,” Wenger said, citing the VITAL trial, led by JoAnn Manson, MD (Brigham and Women’s Hospital, Boston, MA). “They obviously had to enroll a significant number of African-Americans because those are the ones with the major vitamin D deficiency. And as they enrolled, in addition to selectively trying to recruit African-American participants, both men and women, they basically selectively enrolled once certain quotas were reached. They’ve managed to have a trial—and the results have not yet been reported—that has a stunning representation of African-Americans. So there are instances where this can work. It’s not theory.”
It will be years before anyone can say whether the new legislation will improve the gender, race, and ethnicity mix in clinical research only now in the planning stages.
 At the end of my conversation with Wenger, we talked about the ACC’s efforts to improve diversity among the cardiologists on stage at its annual meeting, something Wenger notes the American Heart Association has also been working on as well. I asked: will improving gender/race/ethnicity/nationality mix of cardiology leaders and role models translate into more diversity in clinical trials, since women or men of different backgrounds might be more likely to notice underrepresentation?
At the end of my conversation with Wenger, we talked about the ACC’s efforts to improve diversity among the cardiologists on stage at its annual meeting, something Wenger notes the American Heart Association has also been working on as well. I asked: will improving gender/race/ethnicity/nationality mix of cardiology leaders and role models translate into more diversity in clinical trials, since women or men of different backgrounds might be more likely to notice underrepresentation?
Wenger didn’t miss a beat. This is an idea, she believes, whose time has come.
“I’m hoping that everyone would notice that,” she said. “When you look at a study and you see that no one over age 65 was included, you’re not going to extrapolate that to your elderly patients. And I think we need to have the same sensitivity as we look at the material and methods in [a given study] and ask, do the participants in this study look like the patient sitting in front of me? That will involve many things—that will involve gender, it will involve race and ethnicity, and very importantly it will involve age.” Hopefully over time, she said, “we will get a much more diverse population in the totality of our clinical trials because that really is the evidence base for what we do.”
Find all of TCTMD’s coverage of ACC 2018 on our Conference Page. (Photo credit for images of Dr. Wenger: © ACC/Todd Buchanan 2018. Used with permission.)
Shelley Wood was the Editor-in-Chief of TCTMD and the Editorial Director at the Cardiovascular Research Foundation (CRF) from October 2015…
Read Full BioSources
Wenger NK. Understanding the Journey: The Past, Present and Future of CVD in Women. Presented at: ACC 2018. March 10, 2018. Orlando, FL.
Disclosures
- Wenger reports research grants, contracts, trial steering committee or data safety and monitoring board participation from Gilead Sciences, NHLBI, Society for Women’s Health Research, and consulting for Amgen, AstraZeneca, Gilead, Janssen, and Merck.


Comments