Pascal Device Works Well for Tricuspid Regurgitation in Commercial Market
The transcatheter repair system was safe, efficiently reduced TR, and improved patient function. A pivotal US trial is underway.
Hausleiter J. First commercial multicenter experience with the PASCAL transcatheter valve repair system for tricuspid regurgitation. Presented at: TVT 2021. July 21. 2021. Miami, FL.
MIAMI, FL—The Pascal transcatheter repair system (Edwards Lifesciences) for tricuspid regurgitation (TR) demonstrated promising results in the first multicenter study conducted since it was cleared for commercial use in Europe.
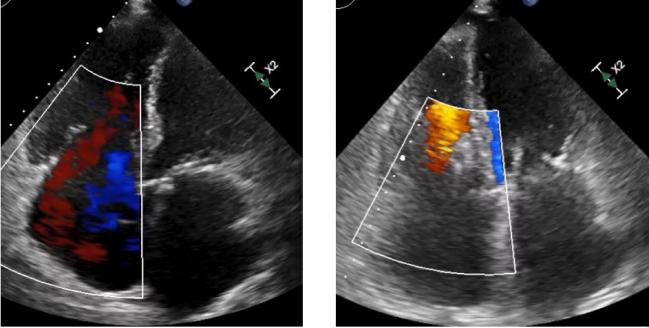
Across four German centers, the device was associated with high technical success and marked improvements in TR. Before the procedure, 92% of patients had more than moderate TR, and that number dropped to 23% at discharge, with the gains sustained through several months of follow-up. TR was reduced by at least one grade in 92% of patients.
Pascal cases were safe, and also led to functional improvements according to NYHA class and 6-minute walk distance. There were indications of RV remodeling and reduced congestion, Jörg Hausleiter, MD (Ludwig-Maximilians-Universität, Munich, Germany), reported yesterday at TVT 2021.
“The efficient TR reduction was durable at follow-up and translated into significant clinical and symptomatic improvement,” he said.
Commenting for TCTMD, Rebecca Hahn, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), one of the TVT course directors, said the findings are similar to what was seen in an early feasibility study she and others published earlier this year.
That’s “always good to see in a commercial setting, because you don’t necessarily have highly trained sites and proctors and things like that,” she said. “It’s always good in a commercial setting to see that the device performance, safety, and efficacy . . . are basically the same as in the trials. It really helps us understand this device and helps to inform this randomized trial that we now have going on in the United States.”
Hahn was referring to CLASP II TR, which is randomizing patients with severe symptomatic TR at an intermediate or greater risk of mortality with tricuspid surgery to transcatheter edge-to-edge repair (TEER) with the Pascal system plus optimal medical therapy or optimal medical therapy alone.
One caveat Hahn mentioned when considering the current results is that the study was conducted at centers with very experienced operators, making it uncertain whether the findings can be generalized to other sites. “But this was a first step, and I think it was a fantastic result,” she said.
Initial Commercial Experience
Technology for transcatheter treatment of TR has been evolving in recent years, and has mostly involved TEER, Hausleiter noted.
The Pascal device received CE Mark approval in Europe in February 2019 for the treatment of mitral regurgitation, with an additional approval for TR announced in October 2020.
Hausleiter and colleagues set out to explore the safety and efficacy of the system after its commercial introduction, examining data on 181 patients with TR (mean age 78 years; 51% women) treated with either the original Pascal device or the next-generation Pascal Ace at four tertiary care centers in Germany. All echocardiographic images were analyzed by a core lab.
The patients had a high surgical risk, as reflected by an average Euroscore II of 5.5% and STS PROM score of 7.7%. They commonly had comorbidities, including CAD (42%), atrial fibrillation (93%), and renal failure (39%). About one-third had an implanted device with a lead through the tricuspid valve.
Technical success was high at 98.4%. Operators used an average of 1.7 devices, procedures lasted an average of 94 minutes, and 9% of patients underwent concomitant mitral TEER. Only five patients had single-leaflet device attachment (SLDA) and two had access-site complications that required intervention—one bleed and one arteriovenous fistula.
Before the procedure, TR was classified as moderate (2+) in 8%, severe (3+) in 38%, massive (4+) in 32%, and torrential (5+) in 22%. At discharge, most patients (77%) had TR graded as moderate or less—33% had only mild regurgitation—and that was sustained through follow-up lasting a median of 147 days. Only 8% of patients had no change in their TR.
Most patients (91%) were in NYHA functional class III or IV at baseline, and that figure fell to 31% during follow-up (P < 0.001). Patients also had an average improvement of 38 meters on the 6-minute walk test (P < 0.001).
Echocardiographic follow-up showed that RV end-diastolic dimension was reduced by an average of 4 mm (P < 0.001) and the size of the inferior vena cava shrank by 3 mm (P = 0.001), reflecting RV remodeling and reduced congestion, respectively, Hausleiter reported.
In terms of clinical outcomes during follow-up, 11.5% of patients died, 15.8% were rehospitalized for heart failure, and 3.6% required a tricuspid reintervention.
Quality vs Quantity of Life
During a panel discussion following Hausleiter’s presentation, Hahn questioned the death rate, noting that a previous presentation with a different transcatheter device for the treatment of TR reported a similar rate but over much longer follow-up.
Hausleiter acknowledged that the mortality rate was not low, pointing out that it was a very sick patient population and that operators are still fine-tuning the selection of the optimal group to treat with the device.
“On the other hand, I think we have to realize that most of those patients are highly symptomatic, and the main goal for those patients is to have an improvement in their symptoms,” he said. “They care less actually if they live a little bit longer, but they want to live better. This is what I’m hearing from our patients.”
To inform guideline recommendations, there will need to be studies demonstrating reductions in mortality and heart failure hospitalizations, but physicians should also keep in mind that patients are also looking for symptom relief, Hausleiter added.
To TCTMD, Hahn said she agreed that most “end-stage patients” are looking for quality and not necessarily quantity of life. “Most of these patients have been ecstatically pleased with a reduction in the early trials of a single grade [of TR],” she said. “A single-grade reduction in the SCOUT trial, for instance, was associated with significant improvements in functional and quality-of-life metrics. And we’ve seen that consistently in all the trials.”
What will also likely be true for the Pascal device, Hahn said, is that reductions in TR will help switch some patients from being unresponsive to diuretics to being responsive. “So now all of a sudden you actually have medical therapy that could also be incorporated into your management decisions,” she said.
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
Hausleiter J. First commercial multicenter experience with the PASCAL transcatheter valve repair system for tricuspid regurgitation. Presented at: TVT 2021. July 21, 2021. Miami, FL.
Disclosures
- Hausleiter reports research support paid to his institution and consulting fees/honoraria from Abbott Vascular and Edwards Lifesciences.
- Hahn reports having relationships with Abbott Vascular, Boston Scientific, and Edwards Lifesciences through her institution and receiving consulting fees/honoraria/speaking fees from Navigate.



Comments