Ultrathin Orsiro Performs Well in All-Comers, Interim CASTLE Data Indicate
The Japanese trial used imaging-guided PCI in order to “clarify the true effect of strut size,” an investigator says.

With imaging-guided PCI, the ultrathin-strut, biodegradable-polymer Orsiro stent (Biotronik) achieves 12-month efficacy similar to what’s offered by the durable-polymer Xience (Abbott) in a wide spectrum patients with stable and unstable CAD, interim results from the CASTLE trial suggest.
Earlier studies, such as BIOSTEMI and BIOFLOW V, have shown the ultrathin-strut Orsiro is better than Xience at reducing the risk of target lesion failure. The question is how. Is it the struts or the polymer? Or is it a difference in how these devices are implanted?
“It has been anticipated that thinner stents have a potential to reduce vessel damage, inflammation, and thrombogenicity,” CASTLE investigator Masato Nakamura, MD (Toho University, Tokyo, Japan), said when presenting the data at EuroPCR 2021. “However, the true mechanism of the superiority of ultrathin-strut DES remains unelucidated,” and there are concerns that thinner stents may offer insufficient radial force in complex lesions, he noted.
Imaging guidance, widely adopted in Japan, “could cancel out differences in the procedure itself and clarify the true effect of strut size,” Nakamura suggested, noting that this “reveals the clinical benefits of advances in stent technology.”
Christopher Cook, MBBS, PhD (Imperial College, London), one of the discussants during the session at which Nakamura presented his findings, commented to TCTMD, “What I think is interesting is that they have attempted to be a little bit pragmatic and use intracoronary imaging as a way of determining whether or not a true difference exists.”
Thanks to reimbursement, almost all PCI in Japan is done with imaging guidance, he pointed out, so clinicians there may be best poised to test this method.
“It’s investigator initiated. . . . It really is a true homegrown study” that speaks to Japanese practice patterns, Cook said, adding that “I think it does add to the literature.”
In particular, he specified that it comes at an opportune time, just after the release at EuroPCR of a meta-analysis confirming an advantage for ultrathin DES. Along with that good news, the analysis also hinted at a potential mortality signal with these devices, one that its authors hope will be clarified by future RCTs as well as longer-term follow-up.
So Far, So Good
CASTLE, an investigator-initiated trial, randomized 1,440 patients (mean age 70 years; ~78% men) at 69 Japanese centers to receive the sirolimus-eluting Orsiro or the everolimus-eluting Xience between May 2019 and March 2020. Clinical and procedural characteristics were similar in the two groups.
Most patients (86%) had chronic as opposed to acute coronary syndromes and around 40% had diabetes. Slightly more than half were treated in the LAD, three-quarters were type B2/C lesions, mean lesion length was approximately 24 mm, two-thirds had a stent diameter ≤ 3 mm, and fully 98% had PCI procedures guided by either OCT or IVUS. Index PCI success was equally good in both groups.
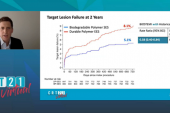
At EuroPCR, Nakamura reported analyses based on approximately 70% follow-up. At 30 days, there was no difference in target lesion failure (CV death, target-vessel MI, clinically driven TLR) between Orsiro and Xience (5.0% vs 4.9%), or in any of the individual components of that outcome. There were no instances of stent thrombosis. For the primary endpoint of TLF at 12 months, which met noninferiority criteria, there also was no difference between the two stents (HR 0.59; 95% CI 0.26-1.36).
“At this moment, [we have] 70% follow-up, therefore cannot say any more. However, we can say numerically lower TLF was observed in the Orsiro group,” driven by target-lesion revascularization, he said, adding, “The final results will be available soon.” CASTLE’s planned follow-up will extend through 24 and 36 months.
Davide Capodanno, MD, PhD (University of Catania, Italy), told TCTMD that CASTLE is “one the many studies that uses a noninferiority design to compare stent A versus stent B. And of course one of the concerns is the ‘biocreep’ phenomenon that we may [encounter] in these situations, where everything is noninferior to another stent and then you go down, down, down.”
That said, he continued, the consistency of results for Orsiro across trials is “very reassuring.”
“Essentially the rate of events is always the same, so I think we should take this collectively and not look it at as a single trial,” but rather as part of the body of evidence showing Orsiro is a safe stent with postive findings in several RCTs, said Capodanno.
‘Elegant Design,’ but Not Definitive
Dejan Milasinovic, MD (Clinical Center of Serbia, Belgrade), in a designated “fact-checking” segment after Nakamura’s presentation, combed through the methodology of CASTLE, which he said has “really an elegant design.”
“When you’re comparing stents, basically what you are looking for is to see what is the impact of stent technology on stent-related events. However, stenting technique might play a confounding role,” he said. “So if you introduce imaging as a sort of standardization to take out the role of stenting technique, this [evens the playing field].”
It’s important to keep in mind, though, that these stents differ not only in terms of strut thickness but also in their polymers and drugs, he added.
Another word of caution, said Milasinovic, is that lower-than-expected event rates—CASTLE had assumed a 12-month TLF rate of 6.3% for Orsiro and 7.2% for Xience—can decrease statistical power and bias toward noinferiority; here, the margin had been set at an absolute difference of 3.3%.
Alexandra Lansky, MD (Yale School of Medicine, New Haven, CT), discussing the results, also highlighted CASTLE’s attempt to reduce variability by way of imaging guidance as unique. With these interim results from CASTLE, which suggest noninferiority but capture only 70% follow-up at 1 year, “we need to be cautious,” she said, adding that even now, though, there appears to be a numeric difference favoring Orsiro.
Nakamura said that at this point the group has actually reached 99% follow-up; it shows a 1-year TLF rate of 5.9% with Oriso and 6.1% with Xience.
Cook, for his part, described the CASTLE data as encouraging, particularly the nearly identical procedural success rates for Orsiro and Xience (93.9% and 93.6%, respectively) that help alleviate concerns about radial strength. As a whole, the evidence base suggests “ultrathin-strut DES do indeed seem to reduce the risk of TLF compared with conventional, second-generation, thin-strut DES,” he told EuroPCR attendees.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Nakamura M. A randomised study comparing imaging guided implantation of Orsiro and Xience - CASTLE study. Presented at: EuroPCR 2021. May 20, 2021.
Disclosures
- Nakamura reports receiving personal fees from Daiichi Sankyo, Terumo, Abbott, and Japan Lifeline.
- Cook reports having received fees from Philips and Boston Scientific, as well as a grant from Edwards Lifesciences.




Comments