ELASTA-Clip Plus TMVR Safe, Feasible for Failed M-TEER
With 22 patients, the report is the largest on the topic thus far and offers lessons on how to optimize this complex procedure.
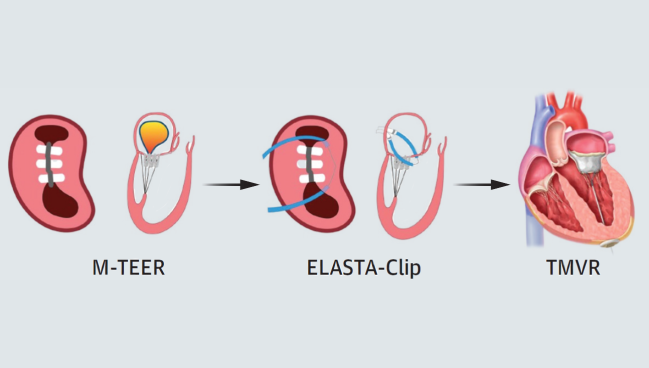
Photo Credit: JACC Cardiovasc Interv. Central Illustration (adapted).
Patients implanted with a mitral clip device that fails over the ensuing months or years can, if appropriately selected, safely undergo a bespoke procedure to detach the clips and then proceed with full transcatheter mitral valve replacement (TMVR), new registry data suggest.
As transcatheter approaches for treating mitral valve disease have evolved, some have raised concerns that prior mitral transcatheter edge-to-edge repair (M-TEER) device placement might preclude surgical replacement.
“Sometimes, in a very small proportion of patients, you can have failure of edge-to-edge repair,” which historically was treated by removing the clips entirely before proceeding with surgical mitral valve replacement, he explained. That isn’t an option for the many patients whose M-TEER procedures were first mandated because they were deemed too high risk for surgery. Repeat M-TEER, as well, is often precluded by the lack of residual mitral valve area or other anatomical factors.
The Electrosurgical Laceration and Stabilization of Clip (ELASTA-Clip) technique, paired with TMVR, is a less-invasive option that widens the pool of patients who could be candidates for reintervention when necessary. With the ELASTA-Clip approach, operators first perform transcatheter electrosurgery to intentionally detach clips from the anterior leaflet, recreating a stable mitral orifice. The technique enables TMVR but leaves the original clips fastened to the posterior leaflet.
Led by Daryoush Samim, MD (Bern University Hospital), the study was published in the February 10, 2025, issue of JACC: Cardiovascular Interventions.
Stroke Rates a Concern
Kashish Goel, MBBS (Vanderbilt University Medical Center, Nashville, TN), speaking with TCTMD, said that in his view, that stroke rate is “unusual [and] very, very high.” A key question is whether the events occurred during the ELASTA-Clip procedure, TMVR, or in relation to some other factor.
He agreed, though, that “there is definitely a need” for ways to address M-TEER failure, and noted that these results for the ELASTA-Clip technique are promising overall. Increasingly, as a field, “we are pushing the limits on M-TEER for patients who are not the perfect anatomical candidates but have no other options, so we’ll do it,” but then there are future consequences that must be dealt with, said Goel.
Hopefully, he added, it will eventually be possible to identify from the outset which patients are best suited to M-TEER and which would fare better by going directly to TMVR.
Paolo Denti, MD (University Hospital Istituti di Ricovero e Cura a Carattere Scientifico Ospedale San Raffaele, Milan, Italy), in an accompanying editorial, points out that more than 250,000 M-TEER cases have been done to date. “Unfortunately, mid/long-term M-TEER failure should be expected if younger patients with more complex anatomies and longer life expectancy are treated,” he writes, “especially in lower-volume centers with less experienced operators in transcatheter therapies.”
Real-world recurrence of mitral regurgitation in M-TEER patients is around 6-10% at 1 year, says Denti.
Less Regurgitation, Better Function
Samim and colleagues enrolled 22 patients (mean age 77.8 years; 40.9% female), all at high surgical risk (mean EuroSCORE II 8.0 and mean STS 7.2%), across eight tertiary care centers in the United States and Europe. Most of those patients had symptomatic residual MR ≥ 3+ (n = 21) following M-TEER with MitraClip (Abbott Vascular) or Pascal (Edwards Lifesciences), while one had iatrogenic mitral valve stenosis. All were ineligible for placement of another M-TEER device.
Comorbidities were common, with 81.8% of patients having arterial hypertension, 68.2% chronic kidney disease, 50.0% atrial fibrillation, and 50.0% coronary artery disease. Ten (45.6%) had previously undergone cardiac surgery.
ELASTA-Clip plus Tendyne (Abbott) implantation was performed at a median of 25.5 months after M-TEER. The ELASTA-Clip technique was done via a transseptal approach in 90.9% and transapical in 9.1%, followed by transapical implantation of the TMVR device—all cases were successful. Technical success according to MVARC criteria was achieved in all but one case (95.4%), without any left ventricular outflow tract obstruction or conversion to sternotomy.
For 45.5% of patients, most of whom had several clips detached, ELASTA-Clip followed by TMVR was performed under elective hemodynamic support using an intra-aortic balloon pump. Periprocedural stroke occurred in two patients (9.1%), with one being disabling and the other nondisabling; neither had cerebral protection during their procedure. The rate of major/life-threatening bleeding was 13.6% and the rate of major access-site vascular complications 9.1%.
At hospital discharge, NYHA functional class significantly improved to II or better in 81.3% and all had residual MR grade 1+.
By 30 days, three patients (15%) had progression of paravalvular leak and an additional ischemic stroke had occurred, bringing the total to three strokes. Three patients (15%) were rehospitalized for heart failure, with two of those cases involving uncontrolled atrial fibrillation and one involving reintervention. However, fully 95% saw their baseline MR reduced to grade ≤ 1+ and none had died.
Median follow-up duration in the study was 8.5 months, with durable MR reduction seen in 89.5% and NYHA functional class of II or less in 72.2% at final follow-up. Among the 13 patients with 1-year follow-up, four (30.8%) died during that span, with three of those deaths due to noncardiovascular causes.
Denti, in his editorial, concurs with the assessment that “ELASTA-Clip and transcatheter mitral valve implantation with Tendyne are feasible and can be performed with acceptable results in a carefully selected patient population.
However, he continues: “Paramount importance must be dedicated to mitigating the risk of cerebrovascular events. Another important point is to reduce the incidence of patients with recurrent MR with either the evolution of actual devices or by adding a reliable tool for percutaneous annuloplasty that can maintain a better result longer and be used in a landing zone.”
Looking to the Future
Praz stressed that selection is crucial. Patients need to not only have suitable anatomy for the ELASTA-Clip approach, but also have the right risk profile in terms of comorbidity burden, age, and frailty level such that they can safely undergo TMVR, which despite being transcatheter still requires surgical cutdown. He pointed out, for example, that patients in their study were “rather young” on average compared with the typical MitraClip population.
Going forward, it will be important to track long-term outcomes of this technique as well as to better understand predictors, prevention, and early detection of failed M-TEER, said Praz.
Regulatory hurdles remain. In Europe, TMVR using the Tendyne device has approval only when transapical. In the United States, Goel pointed out, the Food and Drug Administration has yet to sign off on TMVR, so these procedures are done on a compassionate use basis.
Moreover, this is just one of several methods being explored. Another, though still in its early days, is to not just detach the clips but remove them entirely before then performing TMVR, said Praz, adding that this might reduce stroke risk.
“There are some dedicated devices coming that will remove the clip,” Goel said, “and obviously that will make it much cleaner and easier going forward. . . . When those things come, it will be an interesting way to deal with [M-TEER failure].” And while outcomes with ELASTA-Clip/Tendyne are good, devices designed for transseptal TMVR also could make repeat procedures safer, he said.
Caitlin E. Cox is Executive Editor of TCTMD and Associate Director, Editorial Content at the Cardiovascular Research Foundation. She produces the…
Read Full BioSources
Samim D, Sorajja P, Lanz J, et al. Transapical transcatheter mitral valve replacement after failed transcatheter edge-to-edge repair: a multicenter experience. JACC Cardiovasc Interv. 2025;18:311-321.
Denti P. Lifelong M-TEER patients’ management. JACC Cardiovasc Interv. 2025;18:322-324.
Disclosures
- Samim has received funding for an online course from Edwards Lifesciences.
- Praz was compensated for travel expenses by Abbott Vascular, Edwards Lifesciences, Medira, inQB8 Medical Technologies, and Siemens Healthineers.
- Goel reports serving as a consultant to Edwards and Abbott.
- Denti has received speaker honoraria from Abbott and Edwards Lifesciences; and is a consultant for Pi-Cardia, Approxima, Innov-Heart, and HVR.





Comments