LAA Closure Feasible in Patients With Thrombus: Registry
Presence of thrombus in the LAA is relatively common in AF patients, but considered a contraindication to closure.

For patients who are scheduled for left atrial appendage (LAA) closure but have documented thrombus in the appendage, directly proceeding with their procedure does not translate into a higher risk of major adverse events when compared with receiving an intensified course of antithrombotic therapy prior to closure, according to the results of a new study.
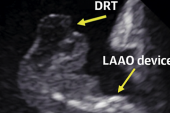
“Of concern, we did find a high rate of device-related thrombus during follow-up, around 13%, which is higher than has been described in previously reported randomized trials and registries,” said lead investigator Gabriela Tirado-Conte, MD (Hospital Clínico San Carlos/Instituto de Investigación Sanitario, Madrid, Spain). Tirado-Conte presented the data as a “Key Abstract” online last week as part of a sneak peek at TCT 2021.
There was no significant difference in the rate of device-related thrombosis between those who underwent direct percutaneous LAA closure and those who received intensified antithrombotic therapy prior to the procedure. In the entire cohort, device-related thrombosis was observed in 14.7% of patients who did not receive oral anticoagulation after LAA closure compared with 4.0% of those who did.
Given the high rate of device-related thrombosis in these patients, Tirado-Conte said they recommend close clinical and imaging follow-up. As for the reason the rate was so high, she pointed out that 47.0% of all patients were treated with anticoagulation at baseline while another 15% were treated with anticoagulation plus a single antiplatelet agent.
This suggests “there may be some kind of prothrombotic environment in this population,” said Tirado-Conte. Further study will be needed to tease out the true extent of the risk and the best treatment approach.
Thrombus in the LAA
LAA closure is indicated for stroke prevention in select patients with atrial fibrillation but the presence of thrombus in the LAA, which is relatively common in patients with AF, is a contraindication to the procedure. Instead, the recommended treatment is the intensification of antithrombotic therapy. However, Tirado-Conte said that LAA closure is being considered off-label in some centers because of the risk of bleeding and incomplete thrombus resolution with intensified antithrombotic therapy.
“The management of LAA thrombus is a challenging situation due to the high bleeding and ischemic risk that usually coexist in patients referred for left atrial appendage closure,” she said.
In this retrospective, multicenter registry, 121 patients were referred for LAA closure and found to have thrombus in the appendage on preprocedural imaging. Of these, 53 underwent direct LAA closure while 68 patients had the procedure deferred until after they were treated with intensified antithrombotic therapy.
Those who were treated with direct LAA closure were older (74.7 vs 70.9 years; P = 0.04), were more likely to have a prior history of bleeding (77.4% vs 60.3%; P = 0.04), had higher HASBLED scores (3.6 vs 3.0; P = 0.01), and were more likely to have an absolute contraindication to oral anticoagulation (45.3% vs 21.9%; P = 0.005). Additionally, those treated with direct LAA closure were more likely to have thrombus in the apex of the appendage and less mobile thrombi.
For those treated with the intensified antithrombotic strategy, 67.1% underwent parenteral anticoagulation with low-molecular-weight heparin. After the initial treatment course, complete thrombus resolution occurred in 60.3% of patients, a percentage that rose to 75.3% in patients who underwent multiple rounds of intensified treatment. Bleeding complications were seen in 9.6% of patients treated with the intensified antithrombotic strategy.
The majority of patients were treated with the Amplatzer Amulet occluder (Abbott), particularly among those who underwent direct LAA closure (77.4%). In those first treated with intensified antithrombotic therapy, 54.8% received the Amulet occluder and 28.7% were treated with Watchman/Watchman FLX (Boston Scientific). The “no-touch” technique was used in 64.2% of patients who underwent direct LAA closure compared with just 20.6% of cases where antithrombotic treatment was first intensified.
In terms of periprocedural outcomes, there was no significant difference between the two groups, with similar rates of device and procedural success, as well as rates of complete LAA occlusion.
“It’s important to say that there were no cases of stroke or systemic embolism during the admission,” said Tirado-Conte.
At 18 months, there was no significant difference in the risk of major adverse events (bleeding, death, or stroke) between the two groups. Rates of bleeding during follow-up were not significantly higher in the group treated first with antithrombotic therapy, but the risk of bleeding was significantly higher in the first 3 months, which represents the time the patients were treated with the more-aggressive course of antithrombotic therapy before undergoing LAA closure.
Poonam Velagapudi, MD (University of Nebraska Medical Center, Omaha), one of the discussants following the presentation, pointed out that while the rate of device-related thrombus was higher than expected, the risk of stroke was not any higher in this patient population. She also questioned whether use of newer-generation technology might help offset the risk. “The use of Watchman was quite low in this group,” said Velagapudi. With the more-recent Watchman FLX there is less exposed metal compared with the original, “so I wonder if the rate of device-related thrombus would be lower,” she hypothesized.
Tirado-Conte said that’s a question they can’t answer but noted that most centers used Amulet as it facilitates the no-touch technique, which minimizes manipulation of the LAA during implantation. Very few patients were treated with FLX, she said, and the no-touch technique is not possible with the original Watchman.
As for the best approach in patients with LAA thrombus detected on imaging, Tirado-Conte said the new registry data show that the direct closure approach is feasible, “but an individualized treatment strategy is required, one that considers the anatomical characteristics and bleeding risk in each patient.”
Michael O’Riordan is the Managing Editor for TCTMD. He completed his undergraduate degrees at Queen’s University in Kingston, ON, and…
Read Full BioSources
Tirado-Conte G, on behalf of the LATOP registry investigators. Management and outcomes of patients with left atrial appendage prior to percutaneous closure. Presented at: TCT 2021. October 6, 2021.
Disclosures
- Tirado-Conte reports no conflicts of interest.




Comments