Tricuspid Repair With Pascal Safe, Effective at 1 Year: CLASP TR
Imaging technology will have to grow alongside procedural acumen for TEER to be successful, said Athena Poppas.

WASHINGTON, DC—Transcatheter tricuspid valve repair with the Pascal system (Edwards Lifesciences) significantly reduces tricuspid regurgitation (TR) with durable outcomes at 1 year, according to new data from the early feasibility CLASP TR study.
The findings—which show 88% survival and 79% freedom from heart failure hospitalization at 1 year—build off successful results seen with transcatheter edge-to-edge repair (TEER) with PASCAL at 30 days and 6 months, as well as in commercial use.

“The take-home message is that tricuspid regurgitation is bad for you, and we don’t have therapies right now for it,” said Adam Greenbaum, MD (Emory University Hospital, Atlanta, GA), who presented the results today at the American College of Cardiology (ACC) 2022 Scientific Session, in a press briefing. This study “hints that we are helping these people, and we need more data from a large, randomized trial to confirm it.”
Commenting on the study, Megan Coylewright, MD (Erlanger Health System, Chattanooga, TN), said, “If you are a patient with severe tricuspid regurgitation or you have a family member that has severe tricuspid regurgitation, this is a very exciting early feasibility study.”
Prior to this and a handful of other new transcatheter options being developed to treat TR, she explained, patients were typically given diuretics, “and it led to hospitalizations and RV death. We didn’t offer surgery. It was deemed to be too high risk. We only did about 5,000 isolated tricuspid valve surgeries over a 10-year period. It’s not a surgery you offer very often, so it is so important to have new choices for patients.”
“As an echocardiographer, I agree,” said Athena Poppas, MD (Lifespan Cardiovascular Institute and Brown University, Providence, RI), who moderated the press briefing. “To do a study like this is not easy and to do some of the imaging in these patients is challenging.”
Likewise, Michael Reardon, MD (Houston Methodist, TX), also in the press briefing, said he, too, is excited to see a new option for tricuspid disease, commenting: “One of my biggest things as a surgeon is: I don’t want to do this, let’s get this fixed.”
Findings at 1 Year
One-year findings for CLASP TR were very similar to earlier data. Among the 46 patients of the original 65 enrolled with complete follow-up, the composite major adverse event rate was 16.9%, with most events being bleeding (9.2%). There were three strokes, one reintervention related to the device, two major access-site and vascular complications requiring intervention, and five patients died of cardiovascular causes.
Greenbaum highlighted that most adverse events were early and none of the deaths were linked to the clasp or procedure. “There were no late complications from the device,” he said.
While patient follow-up was made challenging due to the pandemic, a paired analysis of available data showed all had improved by at least one NYHA grade at 1 year, with 75% improving by at least two grades and 86% reaching moderate or less (P < 0.001). Additionally, researchers observed significant improvements in Kansas City Cardiomyopathy Questionnaire score (P < 0.001) and 6-minute walk distance (P < 0.014).
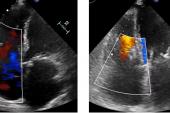
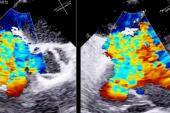
Echocardiographic data also is “favorable,” Greenbaum said, with improvements from baseline to 1 year in tricuspid annulus diameter, RV end-diastolic diameter, RA volume, inferior vena cava diameter, and TR jet area (P < 0.013 for all).
Imaging Needs Progress, Too
Greenbaum noted some of the limitations of doing a feasibility study like this in that “early on, when there were people with massive and torrential regurgitation being enrolled, we needed to understand which patients might benefit from the procedure, how to perform the procedure, [and] how to image the procedure,” he said, adding that procedural time decreased over the length of the study.
Additionally, though imaging the tricuspid leaflets has presented a challenge because they are thinner and also positioned further from the TEE probe, Greenbaum said new technology like intracardiac echo and 3D probes is allowing for smoother procedures.
To TCTMD, Poppas said she is “very encouraged” by the advancements in imaging that are enabling these procedures, but cautioned that operators “can't just start doing this tomorrow. You've got to get all of the pieces around it—the technical skills, the imaging skills, the patient selection, [and also] having a little more focus on perhaps intervening a little earlier in some of these patients.”
The imaging will have to grow alongside the technical aspects of tricuspid valve repair in a “leapfrog” fashion, Poppas added. “The interventionalist may learn the clip skills on the left side and transfer it to the right, but the imagers need to be able to do the same, and the tricuspid valve imaging is challenging.”
Laying Out the Potential Options
As far as where TEER might fit in in the future alongside surgical and transcatheter tricuspid valve replacement, Greenbaum said “we need to learn a little bit more about who is going to benefit from these therapies. . . . I think we need to be treating these people earlier, [because then] surgical outcomes would be better. I also think transcatheter procedures would be easier.”
He pointed to the fact that the patients in CLASP TR was conducted in selected patients. “So there's no doubt that we have learned more about who would benefit from which type of therapy and surgery, but if you look at this select population, it looks like there's some benefit,” he said. “It's durable out to 1 year with associated improvements in functional class, so that's encouraging. Do we need to get everybody down to moderate or less? We need more data.”
The deaths observed in the study are most likely due to the high comorbidity burden of patients with severe TR, according to Greenbaum, who said the mortality seen here is similar to what has been seen with tricuspid valve replacement with Evoque (Edwards Lifesciences). “Dying is not a complication from the procedure; they are dying from their underlying illness,” he said. “These patients still have heart failure. They have other disease. They may not have had severe left-sided disease, but they certainly have some, and I think we need to better be able to select who is unlikely to benefit from the procedure. I think that's going to come with larger trials.”
This is one of the reasons the population of patients with severe TR is so hard to treat, Poppas said. “One reason we haven't done a lot for them is they're either coming too late or it's a marker for prognosis,” she said. “So the interesting thing will be to see if we're going to change their course: is severe TR a marker of end-stage disease or can fixing them change their trajectory?”
Poppas said she is also curious to know whether a percutaneous approach will change symptoms in the short term, “because patients may want quality rather than quantity of life to be able to do more and feel better.”
“Having all the options will be important,” Coylewright told TCTMD. “Some may not be a candidate for repair, may be for replacement, and vice versa. So I think in this area right now, we want to ensure that patients have the most access possible given that surgery is not a traditionally sought after interaction for severe TR.”
With the Pascal device and TriClip (Abbott) being evaluated for TEER, she said she’s “hopeful that we'll see some benefit in the larger pivotal trials and be able to have that available.” However, Coylewright added, “there will still be patients that are not candidates for those therapies that will need access to transcatheter valves.”
“We know from decades of surgical work that replacing a surgical valve is never as good as repairing a surgical valve, ever, ever, ever,” Reardon added. “The question is: does clipping the tricuspid act more like a surgical repair or valve replacement? If it acts more like a repair, and then it works well, that's clearly going to be the front line.”
Yael L. Maxwell is Senior Medical Journalist for TCTMD and Section Editor of TCTMD's Fellows Forum. She served as the inaugural…
Read Full BioSources
Greenbaum A. Transcatheter treatment of tricuspid regurgitation: one-year results of the CLASP TR study. Presented at: ACC 2022. April 4, 2022. Washington, DC.
Disclosures
- Greenbaum reports serving as a proctor for Edwards Lifesciences and Medtronic; holding equity in Transmural Systems; and institutional research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices with Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific.


Comments